
Navigating the sophisticated landscape of FDA polices can be a critical problem for professional medical gadget corporations striving to bring progressive products and solutions to marketplace. E & E Medicals & Consulting stands being a dependable companion, giving specialised know-how in FDA regulatory intelligence and compliance to make certain seamless market place entry and sustained compliance. Which has a deep understanding of the regulatory surroundings, E & E Medicals & Consulting empowers corporations to deal with the intricate requires of the U.S. Meals and Drug Administration (FDA) effectively and properly.
The FDA’s regulatory framework for clinical equipment is multifaceted, encompassing premarket submissions, high-quality system regulations, publish-marketplace surveillance, and labeling needs. Non-compliance can cause high priced delays, merchandise remembers, as well as authorized repercussions. E & E Medicals & Consulting gives tailor-made options to mitigate these dangers, leveraging its complete knowledge of FDA insurance policies, steerage files, and sector ideal methods. By staying abreast of evolving polices and rising developments, the company ensures that shoppers continue being compliant even though optimizing their solution improvement timelines.
Among the Main companies offered by E & E Medicals & Consulting is aid with premarket submissions, for example 510(k) notifications, Premarket Approval (PMA) purposes, and De Novo requests. These submissions call for meticulous documentation and a clear demonstration of basic safety and efficacy. E & E’s group of experts guides purchasers throughout the preparing and submission approach, assisting them craft strong programs that fulfill FDA expectations. This features conducting gap analyses, acquiring regulatory techniques, and making sure alignment with applicable benchmarks, including those through the Intercontinental Firm for Standardization (ISO).
Further than premarket help, E & E Medicals & Consulting excels in encouraging organizations build and preserve compliant Excellent Management Techniques (QMS). The FDA’s High-quality Method Regulation (QSR), outlined in 21 CFR Portion 820, mandates arduous controls for style, production, and write-up-marketplace pursuits. E & E helps clients in implementing QMS frameworks that not merely fulfill FDA prerequisites but additionally increase operational effectiveness. This features training on excellent production techniques (GMP), conducting interior audits, and making ready for FDA inspections.
Post-marketplace compliance is an additional essential spot wherever E & E Medicals & Consulting provides benefit. The business allows clients navigate specifications for adverse occasion reporting, Health-related Unit Reporting (MDR), and corrective and preventive actions (CAPA). By proactively monitoring publish-marketplace efficiency and addressing prospective troubles, E & E makes sure that clientele sustain compliance although safeguarding affected person safety and item reputation.
E & E Manufacturing Medicals & Consulting also provides strategic regulatory intelligence, holding shoppers knowledgeable about FDA policy modifications, new guidance files, and enforcement developments. This proactive method permits corporations to anticipate regulatory shifts and adapt their methods appropriately. No matter if it’s addressing exclusive difficulties for novel systems or guaranteeing compliance for set up equipment, E & E’s skills spans the total products lifecycle.
In an industry where by regulatory precision is paramount, E & E Medicals & Consulting serves being a beacon of reliability. By combining complex experience, regulatory Perception, along with a shopper-centric strategy, the business empowers health care gadget providers to realize compliance, speed up industry obtain, and provide Safe and sound, powerful goods to clients globally.
 Josh Saviano Then & Now!
Josh Saviano Then & Now! Hailie Jade Scott Mathers Then & Now!
Hailie Jade Scott Mathers Then & Now!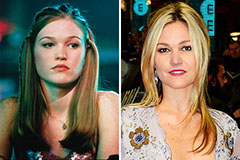 Julia Stiles Then & Now!
Julia Stiles Then & Now! Karyn Parsons Then & Now!
Karyn Parsons Then & Now! Lynda Carter Then & Now!
Lynda Carter Then & Now!