
Navigating the elaborate landscape of FDA restrictions is without doubt one of the most important problems confronted by health care gadget corporations. With continually evolving guidelines, direction files, and submission needs, being compliant is vital—don't just to gain current market access but in addition to be certain individual basic safety. This is when E & E Medicals & Consulting stands out, supplying specialised FDA regulatory intelligence products and services that empower organizations to maneuver ahead with confidence.
What exactly is FDA Regulatory Intelligence?
FDA regulatory intelligence refers to the whole process of accumulating, analyzing, and interpreting regulatory info to assist strategic conclusion-making. For health-related product brands, this involves knowing recent FDA necessities, pinpointing potential dangers, and ensuring that solution advancement aligns with regulatory expectations from day a single.
As an alternative to counting on guesswork or outdated assets, firms need to have up-to-date and actionable intelligence to efficiently navigate premarket submissions, inspections, labeling demands, and put up-market place surveillance. Regulatory intelligence allows proactive compliance, minimizes time to marketplace, and boosts the achievements level of FDA submissions.
E & E Medicals & Consulting’s Tactic
At E & E Medicals & Consulting, the staff delivers deep knowledge along with a tailored method of just about every consumer. Regardless of whether a startup or an established company, E & E supplies very clear, strategic insights into FDA specifications And exactly how they implement in your specific products and organization model.
Their products and services include things like:
Regulatory Method Development: Crafting a regulatory roadmap that outlines quite possibly the most productive path to current market, depending on products classification, meant use, and risk.
Regulatory Investigation and Analysis: Checking changes in FDA restrictions, advice paperwork, and enforcement trends to help you companies keep in advance from the curve.
Submission Guidance: Assisting with 510(k), De Novo, PMA, together with other submission kinds to be sure precision, completeness, and alignment with present FDA anticipations.
Labeling and Marketing Compliance: Making certain product or service promises and promotional resources fulfill FDA requirements and keep away from enforcement steps.
Post-Industry Surveillance: Guiding firms as a result of complaint handling, adverse function reporting, remembers, and FDA inspections.
Why Regulatory Intelligence Issues
Failing to be familiar with or adjust to FDA regulations can lead to high priced delays, warning letters, and perhaps product recalls. Regulatory intelligence isn’t just about staying compliant—it’s about creating smarter enterprise selections. By knowledge the FDA’s anticipations early on, corporations can lower enhancement prices, avoid unnecessary tests, and streamline their route to sector.
What's more, the regulatory landscape is dynamic. With new systems for instance digital wellness apps, AI-driven equipment, and mix solutions rising, the FDA’s stance is constantly evolving. E & E Medicals & Consulting assists customers interpret these modifications and adjust their regulatory system appropriately.
A Trustworthy Companion in Compliance
E & E Medicals & Consulting is more than simply a consulting agency—it’s a strategic spouse dedicated to encouraging clinical system businesses reach a hugely regulated field. That has a dedication to precision, integrity, and results, their FDA regulatory intelligence expert services are intended to assist providers navigate issues, decrease possibility, and accomplish their goals.
No matter if you are producing a fresh medical system or maintaining Manufacturing compliance for an current product, E & E Medicals & Consulting is below to guide you every move of the way in which.
 Barret Oliver Then & Now!
Barret Oliver Then & Now! Bradley Pierce Then & Now!
Bradley Pierce Then & Now!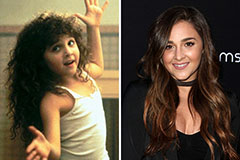 Alisan Porter Then & Now!
Alisan Porter Then & Now! Hailie Jade Scott Mathers Then & Now!
Hailie Jade Scott Mathers Then & Now! James Van Der Beek Then & Now!
James Van Der Beek Then & Now!