
Navigating the complicated landscape of FDA regulations is usually a essential obstacle for health care gadget corporations. E & E Medicals & Consulting stands like a reliable companion, presenting specialised knowledge in FDA regulatory intelligence and compliance that will help organizations convey Secure, helpful, and compliant goods to market place. Which has a deep understanding of the regulatory setting, E & E Medicals & Consulting presents tailored answers that streamline procedures, mitigate threats, and make sure adherence for the FDA’s stringent criteria.
The FDA’s regulatory framework is multifaceted, encompassing premarket submissions, quality program rules, article-market surveillance, and much more. For health care system corporations, compliance is not merely a lawful obligation but a cornerstone of product achievement. Non-compliance can result in pricey delays, solution recalls, or perhaps enforcement actions. E & E Medicals & Consulting excels in guiding businesses via this intricate procedure, supplying stop-to-stop help that spans solution improvement to marketplace entry and beyond.
One of the Main expert services supplied by E & E Medicals & Consulting is help with premarket submissions, such as 510(k) clearances and Premarket Acceptance (PMA) apps. These submissions call for meticulous documentation, robust scientific facts, and a clear demonstration of safety and efficacy. E & E’s workforce of gurus aids customers get ready detailed submissions, making certain alignment with FDA anticipations. By anticipating likely regulatory hurdles, they reduce the chance of delays and increase the chances of An effective end result.
Further than premarket assist, E & E Medicals & Consulting focuses on High-quality Program Regulation (QSR) compliance, as outlined in 21 CFR Part 820. This regulation mandates that suppliers establish and retain a high quality administration method to guarantee item basic safety and efficiency. E & E helps clients in establishing, implementing, and auditing top quality units that satisfy FDA necessities. Their proactive method will help detect gaps, deal with deficiencies, and foster a lifestyle of continual improvement.
Put up-sector compliance is another vital place where E & E Medicals & Consulting shines. The FDA needs ongoing vigilance by adverse party reporting, item labeling compliance, and put up-current market surveillance studies. E & E aids firms create strong units to observe product functionality, reply to adverse situations, and maintain compliance with labeling and promoting rules. This makes certain that providers remain in great standing With all the FDA even Manufacturing though safeguarding client protection.
In addition to specialized knowledge, E & E Medicals & Consulting delivers strategic regulatory intelligence. By keeping abreast of evolving FDA procedures, steering files, and field trends, they provide customers with actionable insights to navigate regulatory modifications. This forward-thinking solution allows firms to adapt swiftly, no matter whether responding to new cybersecurity needs or incorporating digital health and fitness systems into their units.
E & E Medicals & Consulting’s client-centric solution sets them aside. They tailor their companies to meet the unique requires of every organization, whether or not a startup launching its first product or an established manufacturer increasing its portfolio. Their collaborative procedure fosters belief, transparency, and measurable results.
Within an market where regulatory compliance may make or break an item, E & E Medicals & Consulting is a vital ally. Their skills in FDA regulatory intelligence empowers healthcare product organizations to achieve compliance, accelerate sector entry, and provide ground breaking remedies that improve client outcomes.
 Lark Voorhies Then & Now!
Lark Voorhies Then & Now! Judge Reinhold Then & Now!
Judge Reinhold Then & Now!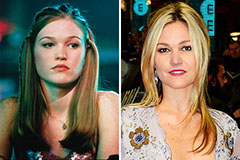 Julia Stiles Then & Now!
Julia Stiles Then & Now! Barbi Benton Then & Now!
Barbi Benton Then & Now!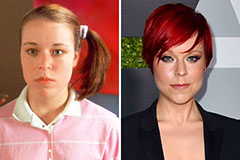 Tina Majorino Then & Now!
Tina Majorino Then & Now!